Hypertension
Download: Hypertension PDF
Background
Hypertension complicates approximately 10% of all pregnancies and is a leading cause of maternal and perinatal morbidity and mortality nationally and internationally. The most important aspect in the approach to these disorders is to be able to recognize when high blood pressure is due to an ominous disease process such as preeclampsia, versus a hypertensive disorder that antedates the pregnancy. Many societies have developed an approach to the diagnosis, classification, and management of hypertension in pregnancy, and this discussion will focus on the Canadian guidelines, published in 2008 and endorsed by the SOGC (1).
Diagnosis of hypertension in Pregnancy
The diagnostic criteria for hypertension varies slightly worldwide, but the general acceptance is a diastolic blood pressure (BP) greater than 90 mm Hg, on 2 measurements, at least 4 hours apart. The NHBPEP (2) also includes a systolic blood pressure greater than 140 mm Hg, and the Canadian guidelines suggest following patients closely if they have isolated systolic readings greater than 140 mm Hg. Previous criteria included changes in blood pressure (for instance changes in systolic greater than 30 mm Hg), but this has been progressively abandoned because of the normal physiologic changes of pregnancy. Systemic vascular resistance falls in the fist half of pregnancy and then rises in the third trimester; therefore significant changes in BP over the course of a pregnancy may be entirely physiologic. Severe hypertension is defined as systolic readings greater than 160 mm Hg. It should be noted that this limit has been progressively falling over time because of the realization that risk of stroke rises with readings greater than 160 mm Hg, rather than 180 mm Hg.
Blood pressure should be measured with the patient in the sitting position, with the arm supported at the level of the heart using an appropriate sized cuff. Korotkoff phase V, ie, disappearance of sounds should be used. In some patients, muffled sounds can be heard almost to 0 and in that case, phase IV (or muffling) can be used to guide diagnosis and treatment.
Classification of hypertensive disorders in Pregnancy
Hypertensive disorders in pregnancy should be classified by the underlying cause.
Preexisting hypertension
Preexisting hypertension is the term used in high blood pressure is present before pregnancy. Because of the expected physiologic drop in BP in the first half of pregnancy, women who have diastolic BP greater than 90 mm Hg before 20 weeks gestation are also likely “previously unrecognized” hypertensives.
Gestational Hypertension
Gestational hypertension develops after 20 weeks gestation. This term indicates that the main etiology of the hypertension is related to pregnancy, in contrast to preexisting hypertension. It’s an important distinction as in preexisting hypertension; high blood pressure is the only pathology, whereas in gestational hypertension it is only a manifestation of an underlying systemic process. In addition, gestational forms are expected to eventually resolve once the pregnancy is over. This group of disorders includes both non-proteinuric hypertension (often referred to as “gestational hypertension”), and proteinuric hypertension, which is in effect preeclampsia.
Non-proteinuric gestational hypertension
This should almost be considered as “pre-preeclampsia”, since 40% of these patients will eventually meet criteria for it. The rise in BP is often the first indication that a patient is about to manifest the syndrome and these patients certainly need to be followed closely. If the hypertension is severe, then fetal outcomes are actually quite similar to preeclampsia, once again highlighting the close relationship between the two, and perhaps the over-dependence on proteinuria as a diagnostic criterion for preeclampsia. The hypertension resolves postpartum by 6 weeks.
Preeclampsia (ie, proteinuric gestational hypertension)
This is the most ominous subtype of hypertensive disorder in pregnancy. It has been referred to as the “disease of theories”, and that still remains true today. The pathophysiology has not been fully elucidated, although one of the most accepted theories is that of the “2-stage model”. The first stage involves abnormal placentation: placentas of preeclamptic patients reveal inadequate invasion of maternal spiral arteries by the cytotrophoblast cells. This should occur between the end of the first trimester and 20 weeks gestation, and may be due to defective trophoblast differentiation. Regardless, this sets up a process of placental hypoperfusion, hypoxia, and ischemia. The hypoxic placenta secretes factors that initiate the second stage, which is a multi-system process that is clinically recognized as preeclampsia. The actual agents remain elusive, although recent observations suggest that an imbalance in angiogenic factors may be involved. However, it is a complex process, with likely many pathways that lead to abnormal placental development since there are other risk factors such as genetics and immunologic issues to consider.
Risk factors for preeclampsia can be divided into maternal, fetal and placental factors. Maternal factors include: first pregnancy, positive family history in mother or sister, prior personal history of preeclampsia, extremes of maternal age (age less than 20 or greater than 35), obesity, ethnic groups such as Black or South Asian, chronic medical conditions such as preexisting hypertension, diabetes, chronic renal insufficiency, auto-immune disorders such as lupus and antiphospholipid antibody syndrome. Fetal/placental factors include multiple gestation, fetal hydrops, and molar pregnancy.
The diagnosis of preeclampsia is based on the presence of hypertension and proteinuria. Given the above description, preeclampsia is best conceptualized as a “systemic endothelial activation” syndrome, characterized by hypertension and proteinuria, with the root cause being abnormal placental development. Hypertension and proteinuria are the diagnostic tools to classify the hypertensive disorder, thereby identifying the patient that is at risk of severe morbidity and mortality. Proteinuria should be strongly suspected if the dipstick shows 2+ or more. The 24-hour urine collection remains the Gold standard, with greater than 0.3 g/day protein excretion being abnormal. However, this test is time-consuming and is highly dependant on proper collection. Spot (random) urine sample with > 0.3 mg /mmol of protein/creatinine correlates well with 24-hour urine quantification, and can be very helpful in quickly ascertaining whether significant proteinuria is present. Presenting symptoms included headache, blurred vision, upper abdominal pain, new onset vomiting, and rapid onset swelling.
The complications of preeclampsia can be explained by the generalized endothelial dysfunction. The HELLP syndrome was defined in 1982 to aid physicians in detecting a severe subset of preeclampsia. This syndrome refers to “hemolysis, elevated liver enzymes and low platelets” and complicates 10-20% of preeclampsia. The relationship between HELLP and preeclampsia is a little controversial, because 15% of HELLP syndrome occurs in patients without hypertension or proteinuria; but these two diseases are generally thought to be manifestations of the same disease process. Some patients may present with only elevation of liver enzymes or thrombocytopenia (often referred to as “partial HELLP”). Management includes supportive care with blood products, keeping platelets greater than 50, 000. The severity of thrombocytopenia correlates with local hepatic complications, which are mainly subcapsular hematoma, hepatic rupture, and rarely, hepatic infarction related to ischemic hepatitis. The definitive management is delivery. HELLP syndrome can worsen in the first 48 hours following delivery. There has been some suggestion that dexamethasone can hasten its resolution, but results from randomized control trials are conflicting. This needs to be studied further, and is not recommended to be used routinely.
The central nervous system can also be significantly affected. Eclampsia, refers to seizures and/or coma occurring in the setting of preeclampsia. It is still a cause of maternal morbidity and mortality in developing nations, less so in the industrialized part of the world, complicating 1-3% of preeclampsia. The etiology is thought to be either due to a loss of cerebral autoregulation from severe hypertension leading to over-perfusion, although in other cases is may be related to severe vasospasm. Treatment includes expeditious delivery as well as Magnesium sulfate. Magnesium is also effective in preventing eclampsia (3): patients with severe preeclampsia should receive prophylaxis, unless they have oliguric renal failure (magnesium is excreted renally, and hence these patients are at risk of developing magnesium toxicity). Patients on magnesium require close monitoring of reflexes, urine output and cardio-respiratory status. Magnesium toxicity can be treated with calcium gluconate, or in severe cases, hemodialysis.
The renal lesion from preeclampsia is identified as “glomerular endotheliosis”, with glomeruli becoming enlarged and swollen, leading to impaired glomerular filtration, and proteinuria. In normal pregnancy, the glomerular filtration rate increases by 30-40%, and so the creatinine may in fact still be in upper limit of normal in preeclampsia. Fluid balance can be challenging: although patients may be extremely edematous, they are often intravascularly contracted and may be oliguric temporarily. On the other hand, patients are at risk of developing non-cardiogenic pulmonary edema, and excessive fluid administration can be extremely deleterious.
Prevention of preeclampsia is still being investigated. Several studies have suggested that aspirin 81 mg daily can reduce the risk by 10%. Other studies indicate that ensuring adequate micronutrient intake such as folic acid and calcium may be beneficial. Treatment with anti-oxidants (with vitamin E and C) has been well investigated and do not prevent preeclampsia. Women at particularly high risk of preeclampsia may be referred to high-risk centre, and placental Doppler evaluation may be helpful in predicting the incidence of preeclampsia.
Management of Mild or Moderate Hypertension in Pregnancy
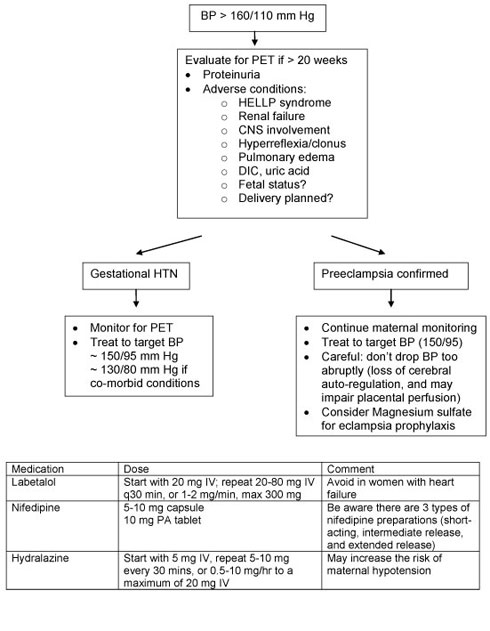
The optimal blood pressure for a pregnant woman is not yet known. Studies seem to suggest that one should avoid either extreme: severe hypertension chronically is associated with fetal growth restriction, whereas acute rise in BP (regardless of the cause) increases the risk of placental abruption. On the other hand, some studies suggest that lowering blood pressure too much is also associated with impaired fetal growth. Canadian guidelines take into account whether there is a maternal reason to be more aggressive in lowering blood pressure; for instance, if there is a “co-morbid” condition such as diabetes, underlying renal disease, or heart disease, target blood pressure should be 130-139/80-89 mm Hg. In the absence of those risk factors, target blood pressure can be anywhere from 130-155/80-105 mm Hg.
As mentioned previously, BP might actually fall in the first half of pregnancy, in which case it is not uncommon to decrease the dose of antihypertensive (or stop it altogether) in women with chronic hypertension in order to maintain their target BP range. BP will often rise in the third trimester, and doses may need to be increased at that point.
Women with preexisting hypertension have a 15-20% risk of developing superimposed preeclampsia and should be informed of the symptoms to watch for, and when to seek medical attention. However, treatment with antihypertensive medication does not prevent it from occurring.
Management of Severe Hypertension in Pregnancy
Women presenting with severe hypertension around 20 weeks gestation should be evaluated for preeclampsia. This includes evaluation of maternal and fetal wellbeing, laboratory investigations, and serial BP monitoring. Treatment of severe hypertension includes labetalol, hydralazine, and nifedipine. Please refer to the algorithm below and table below. Strong consideration should be given to hospital admission for closer observation.
If women are found to have preeclampsia, further management depends on maternal and fetal status, as well as the gestational age at presentation. If the patient is close to term, delivery is beneficial in preventing maternal and fetal complications. However, if the woman is remote from term (i.e. less than 34 weeks gestation), “expectant management” can be considered. This involves delaying delivery until steroids have been administered to allow for fetal lung maturation. Treatment with antihypertensive medication may be necessary, and close monitoring of maternal clinical and laboratory parameters should occur, at least daily. Once stabilized, patients may need to be transferred to a centre with expertise in managing high-risk pregnancies from both a maternal and neonatal perspective.
Pharmacologic management of hypertension in Pregnancy
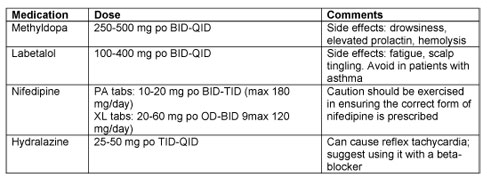
Drugs that do not increase the risk of malformations above the baseline risk of 1-3% in the general population include: methyldopa, labetalol, nifedipine, and hydralazine. Thiazide diuretics also do not increase risk of birth defects, but are often avoided because of theoretical concerns about decreasing circulatory volume. Conception while on diuretics should not be a cause for concern, but these drugs should generally be changed to something that is known to be safer from a fetal perspective.
ACE inhibitors and Angiotensin receptor blockers are known teratogens and should be avoided in pregnancy. Continued use of these agents, particularly during the second and third trimester of pregnancy, leads to fetal renal tubular dysfunction, oliguria, and subsequent oligohydramnios, which in turn can cause limb contractures, and hypocalvaria (inadequate ossification of skull bones). There is some data to suggest that exposure in the first trimester also increases the risk for anomalies and therefore these drugs should be avoided prior to pregnancy in women who have chronic essential hypertension with no end-organ effects or concomitant medical conditions such as diabetes, renal insufficiency, or heart disease. However, in the small group of patients who are attempting pregnancy and who do have underlying renal disease, it may be reasonable to continue ACE-inhibitors until they conceive. It may take months or even years before they are successful in becoming pregnant, and so the risk of maternal long-term risk of discontinuing the drug may outweigh the concerns about exposure in the first trimester. This may need to be evaluated by a physician with expertise in this area.
Postpartum management of hypertensive disorders in Pregnancy
Blood pressure in gestational hypertension often rises in the postpartum period, between days 3-5. These women should have appropriate counseling and follow up arranged before discharge. Preeclampsia and eclampsia can manifest postpartum, and so symptoms of headache, blurred vision, epigastric pain should be evaluated as possible signs of postpartum preeclampsia. Generally, resolution occurs by 6 weeks postpartum, although in severe cases, is may take longer.
Women who develop gestational hypertension have increased risk of chronic hypertension and atherosclerotic disease later in life, and should be encouraged to modify any cardiovascular risk factors and have regular screening for hypertension, dyslipidemia, and diabetes.
References
1) Hypertensive Disorders of Pregnancy, Journal of Obstetrics and Gynaecology Canada 2008; 30(3):Suppl 1 [http://www.sogc.org/guidelines/documents/gui206CPG0803_001.pdf]
2) Report of the National High Blood pressure Education Program working Group on High Blood pressure in pregnancy, American Journal of Obstetrics and Gynecology 2000; 183(1) S1-S22.
3) Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, Smith D; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877-90.
